Topical Administration of G-CSF Ameliorates Refractory Cutaneous Ulcers: A Clinical Case Series
Satoshi Takagi1*, Shiro Jimi2, Takuto Oyama1, Motoyasu Miyazaki3, Junji Suzumiya4, Hiroyuki
Ohjimi1
DOI10.36648/2471-8505.21.7.58
Satoshi Takagi1*, Shiro Jimi2, Takuto Oyama1, Motoyasu Miyazaki3, Junji Suzumiya4, Hiroyuki Ohjimi1
1 Departments of Plastic, Reconstructive and Aesthetic Surgery, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
2 Central Lab for Pathology and Morphology, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
3 Department of Pharmacy, Fukuoka University Chikushi Hospital, Fukuoka, Japan
4 Internal Medicine-Hematology Department, Sankohkai Community Hospital Kohka, Shizuoka, Japan
*Corresponding author: Satoshi Takagi, Department of Plastic and Reconstructive Surgery, Faculty of Medicine, Fukuoka University, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan , Tel: +81-92-801-1011; Email: stakagi@fukuoka-u.ac.jp
Received date: June 6, 2020; Accepted date: August 13, 2021; Published date: August 23, 2021
Citation: Satoshi T (2021) Topical Administration of G-CSF Ameliorates Refractory Cutaneous Ulcers: A Clinical Case Series. Skin Dis Skin Care. Vol. 6 No.2
Abstract
Objective: Granulocyte colony-stimulation factor (G-CSF) is a chemokine that stimulates granulocyte proliferation and maturation and mobilizes bone marrow-derived stem cells into the bloodstream. G-CSF treatment has been shown to enhance tissue repair in various conditions characterized by chronic wounds; however, the mechanisms by which this cytokine promotes chronic wound healing remain unclear. The effect of recombinant G-CSF on wound healing was examined in six patients with intractable chronic cutaneous ulcers.
Methods
G-CSF was topically applied at 6 µg/cm2 over the ulcers daily for 14 days. The wound conditions were assessed using DESIGN-R, a comprehensive scoring system established by the Japanese Society of Pressure Ulcers that monitors ulcers’ severity and healing states.
Results
The mean plasma concentration of G-CSF increased from 34.5 ± 14.1 µg/ml on day 0 to 70.8 ± 61.6 µg/ml on day 7; this increase was positively correlated with that of the WBC count. Total cutaneous ulcers graded with DESIGN-R scores significantly improved on day 7 (p < 0.05).
Conclusions
Topical administration of G-CSF induces amelioration of refractory cutaneous ulcers, probably via flare-up of inflammation without any damaging side effects.
Keywords: Topical administration, granulocyte colony-stimulation factor, refractory cutaneous ulcers, wound healing.
Introduction
Skin wound healing is a multi-stage process that involves the reestablishment of haemostasis, inflammation, cell proliferation, and tissue remodelling [1]. Inflammation is a crucial stage in this process. It is an essential initial reaction against injury and infection and is necessary for the removal of damaged tissues and pathogens. Inflammation is also required for the preliminary preparation for granulation, neovascularisation, and regeneration [2]. Monocytes, macrophages, and neutrophils recruited to the site of injury play a key role in not only phagocytosis but also remodelling in wound healing. Non-healed chronic wounds frequently stagnate the sequential healing cascade because of different exacerbating factors pre-existing in the patients [3]. Some of these factors include vascular insufficiency, diabetes, malnutrition, aging/senescence, and local factors including excessive compression, infection, and oedema. Such wounds are often stuck at the chronic inflammation stage and are precluded from entering the next stage—proliferation.
Tissue regeneration requires the proliferation of tissue-specific stem cells residing in not only individual tissues but also afield organs. Being rich in different types of stem cells, the bone marrow is also a potential source of mesenchymal stem/progenitor cells for local tissue repair. Some of the cells involved include endothelial progenitor cells (EPC) [4] and fibrocytes [5]. During inflammation, the granulocyte colony-stimulating factor (G-CSF) as an inducible robust chemokine that helps in mobilizing bone marrow-resided granulocytes and stem/progenitor cells into the bloodstream [6]. After an injury, G-CSF can be produced by damaged tissues to act as an initiating factor for the wound healing cascade [7]. In clinical settings, G-CSF has been successfully utilized to enhance wound healing in neutropenic patients [8, 9], patients with dystrophic epidermolysis bullosa [10] and diabetic foot disorder [11]. However, the mechanisms of action through which G-CSF accelerates refractory wound healing are still unclear.
Here, study authors report the therapeutic and physiologic effects of topical G-CSF treatment in a preliminary clinical trial involving six patients with intractable chronic cutaneous ulcers.
Materials and Methods
Study design
Following approval by the Ethics Committee for Clinical Study (ECCS) at Fukuoka University Hospital (IRB06-44), Fukuoka, Japan, G-CSF-chronic wound intervention trials (TRFU-CSF-01) were performed. Written informed consent was obtained from all participating patients or guardians in accordance with the Declaration of Helsinki.
The Phase 1 clinical trials aimed to determine the safety and efficacy of Filgrastim, or GRAN®, a recombinant human G-CSF (rhG-CSF) provided by Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan. The drug was administered to patients with cutaneous ulcers in refractory stages. Assessments were performed by clinical research physicians from the Department of Plastic and Reconstructive Surgery at Fukuoka University Hospital. The DESIGN-R scoring system, established by the Japanese Society of Pressure, was utilized for the comprehensive evaluation of ulcer stages and its healing process. The categories in DESIGN-R are as follows: ulcers: depth (0-5 points); exudate (0-6 points); size (0-15 points), inflammation/infection (0-9 points), granulation (0-6 points), necrotic tissue (0-6 points), and pocket (0-24 points) [12]. In all categories except “Depth”, the higher the points mean the more serious the individual’s ulcer.
Patients and treatments
Six patients were admitted to the study using the following criteria: 20-75 years old with an ulcer that never improved two weeks before the study; without wound infection; wound less than 12 cm in diameter at its longest end; not invasive into muscles, tendons, or bones, but remained subcutaneously.
Filgrastim at a dose of 6 µg/cm2 ulcer area was topically and administered daily for 14 days. The following agents were not allowed: antibiotics; reagents for granulation tissue development; basic-fibroblast growth factor (bFGF); heparin-like compounds; corticosteroids for topical use; and wound healing systemic administration agents, including prostaglandin-drug formulations, anti-platelet agents, corticosteroids at a dose of more than 20 mg prednisolone equivalent/day, and anti-thrombin agents. The study was ruled to be discontinued by the ECCS if patients developed bacterial infections, an ulcer reaching muscle, tendon, or bone, aggravated colour change, or the appearance of serious adverse effects.
| Patient | Sex | Age | Ulcer lesion | Pre-treatments | Diseases | Comorbidities | DESIGN-R(Day 0) |
|---|---|---|---|---|---|---|---|
| 1 | Male | 63 | Sacral | DebridementNPWT | CI | Type 2 DMBronchial asthma | 12 |
| 2 | Male | 41 | Dorsal | Povidone-iodine | SCI | None | 15 |
| 3 | Male | 74 | Sacral | Iodine-coatOintment | CISCI | Type 2 DM | 14 |
| 4 | Female | 76 | Sacral | Povidone-iodine | SCI | Autoimmune hepatitis | 10 |
| 5 | Female | 72 | Hip (right) | DebridementProteolytic-ointment | CH | None | 14 |
| 6 | Male | 54 | Ischial | Povidone-iodine | SCI | Ossification of posterior longitudinal ligament | 15 |
Table 1: Patient data.
CH: cerebral haemorrhage, CI: cerebral infarction, DM: diabetes mellitus, NPWT: Negative pressure wound therapy, SCI: spinal cord injury.
Patient profile and ulcer assessment
The patients’ profiles are listed in Table 1. All patients received the same standard wound care, and debridement was performed on patients 1 and 5 before Filgrastim administration. None of the patients had wound infections. The DESIGN-R score evaluation was done just before the study and on days 7 and 14 of the study. The total score, excluding the depth score, were calculated (maximum 66 points). Lesion severity is categorized into three levels – 9 or lower: mild; 10-18: moderate; 19 or higher: severe. The initial total DESIGN-R scores for the patients were 12, 15, 14, 10, 14, and 15 points, indicating that they were all at the moderate level (Table 1).
Laboratory analysis
Laboratory analysis was carried out just before the study (day 0) and on days 7 and 14. G-CSF plasma levels were measured using a sandwich ELISA with a human G-CSF antibody (R&D Systems, Inc., MN, USA). Haematological examinations were performed to determine the number of red blood cells (RBC), white blood cells (WBC), platelets, neutrophils, and CD34+ cells in the blood, and the levels of haemoglobin, and haematocrit. Blood levels of AST, ALT, LDH, Al-p, γ-GTP, CRP, total protein, total cholesterol, urea nitrogen, blood glucose, creatinine, and uric acid were also measured. Urinalysis was performed to determine protein, sugar, and urobilinogen levels.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical analysis was performed using ANOVA and regression analysis. A p-value of less than 0.05 was considered to be statistically significant.
Results
Changes in plasma G-CSF level
Table 2 shows the summarized laboratory data. The initial G-CSF plasma level in the patients ranged from 13.8 to 50.5 µg/ml (34.5 ± 14.1 µg /ml). With daily administration of Filgrastim, plasma G-CSF level on day 7 was 70.8 ± 61.6 µg /mL, and 36.1 ± 17.3 µg/mL on day 14. Plasma G-CSF level increased in all patients on day 7, except for patient 5, but mostly returned to the initial level on day 14.
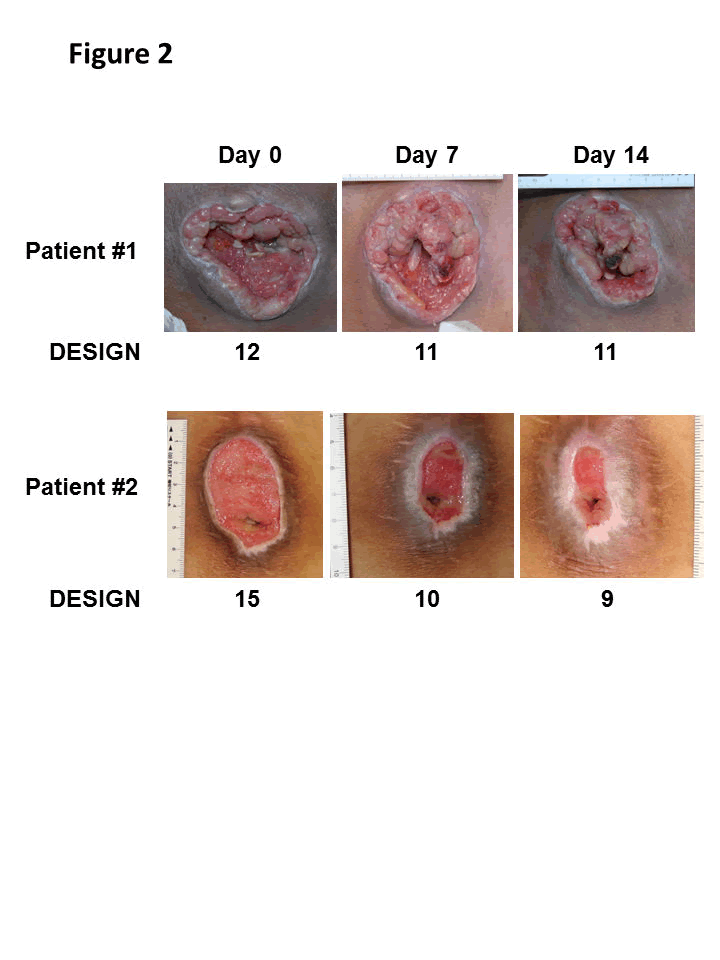
Changes in wounds during the 14 days of G-CSF administration in representative cases (patient 1 and 2) are shown. Both cases healed progressively.
| Patient | Analysis | G-CSF | CD34+ cells | WBC | Haemoglobin | RBC | Platelet | Total protein | CRP |
|---|---|---|---|---|---|---|---|---|---|
| (ng/mL) | (/µL) | (/µL) | (g/dL) | (×104/µL) | (×104/µL) | (g/dL) | (mg/dL) | ||
| 1 | Day 0 | 29.7 | 7 | 6900 | 12.2 | 425 | 34.2 | 6.4 | 1.11 |
| Day 7 | 45.8 | 9 | 10500 | 13.0 | 439 | 34.9 | 6.8 | 2.89 | |
| Day 14 | 31.0 | 7 | 9300 | 13.1 | 454 | 43.1 | 7.2 | 3.89 | |
| 2 | Day 0 | 13.8 | 3 | 5500 | 10.8 | 443 | 33.9 | 7.6 | 0.44 |
| Day 7 | 18.6 | 3 | 8000 | 11.2 | 469 | 40.3 | 7.3 | 0.05 | |
| Day 14 | 17.7 | 3 | 5700 | 10.9 | 459 | 36.3 | 7.1 | 0.12 | |
| 3 | Day 0 | 25.8 | 3 | 6800 | 10.4 | 334 | 18.5 | 7.1 | 0.21 |
| Day 7 | 71.0 | 3 | 6800 | 10.4 | 332 | 16.4 | 7.3 | 0.26 | |
| Day 14 | 24.8 | 7 | 7400 | 10.7 | 342 | 19.1 | 7.3 | 0.19 | |
| 4 | Day 0 | 38.8 | 5 | 5900 | 11.6 | 350 | 14.3 | 6.3 | 0.41 |
| Day 7 | 60.2 | 9 | 7000 | 12.3 | 376 | 14.3 | 6.4 | 0.37 | |
| Day 14 | 53.9 | 4 | 7700 | 11.8 | 358 | 14.1 | 6.2 | 0.30 | |
| 5 | Day 0 | 50.5 | 11 | 9200 | 12.7 | 546 | 34.4 | 7.0 | 0.53 |
| Day 7 | 38.0 | 6 | 6900 | 13.2 | 566 | 28.2 | 7.1 | 0.23 | |
| Day 14 | 28.2 | 5 | 8700 | 13.4 | 566 | 24.2 | 7.3 | 0.12 | |
| 6 | Day 0 | 48.8 | 2 | 3700 | 12.0 | 392 | 20.4 | 7.4 | 4.66 |
| Day 7 | 191.0 | 4 | 10200 | 13.0 | 416 | 26.4 | 8.1 | 1.75 | |
| Day 14 | 61.0 | 3 | 3100 | 13.0 | 425 | 17.8 | 7.8 | 3.11 |
Table 2: Haematological data.
WBC count
WBC count on day 7 increased up to 276% in four cases (patients 1, 2, 4, and 6). In two cases (patients 2 and 6), WBC count returned to around the initial level on day 14 (Table 2 and Figure 1A). Regression analysis indicates a significant correlation between the increase in plasma G-CSF level and the percentage increase in WBC level from day 0 to day 7 (p < 0.05) (Figure 1B). No significant changes in CD34+ cell count and other haematological parameters were observed during the study.
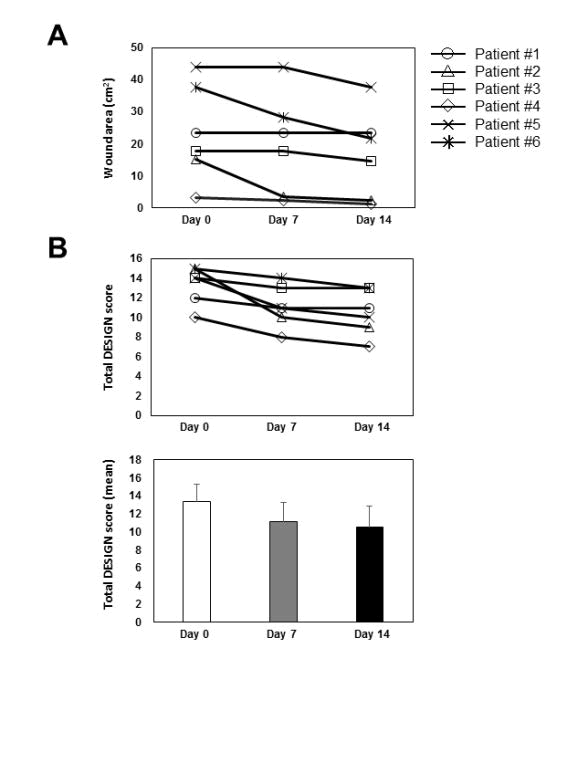
Changes in wound area (A) and total DESIGN-R score (B upper) in all six patients are shown. A significant reduction in the DESIGN-R score was noted from day 0 to day 7 (p < 0.05 by regression analysis), but not from day 7 to 14.
Wound healing and DESIGN-R score
Macroscopically, wounds in all the cases healed progressively (Figure 2). The initial wound area ranged from 3.1 to 44 cm2, with a mean of 23.5 ± 15.1 cm2 (Figure 3A). No significant change was observed in the wound area on day 7(19.9 ± 15.7 cm2; 20.3% reduction vs. day 0), but a significant decrease was observed on day 14 (16.9 ± 13.8 cm2; p < 0.01, 37.5% reduction vs. day 0) (Figure 3A). The mean of DESIGN-R score was initially 13.3 ± 2.0 points and decreased on day 7 (11.2 ± 2.1 points, p < 0.01) and day 14 (10.5 ± 2.3 points, p < 0.01) (Figure 3B). The reduction of 2.2 ± 1.6 points from day 0 to day 7 was statistically significant (p < 0.05 by regression analysis). However, that from day 7 to 14 was not significant (reduction of 0.7 ± 0.5 points). No adverse wounds or systemic conditions were found in all six patients.
Discussion
It is important for physicians to choose a regimen for patients with intractable wounds to allow the shift in tissue stages from inflammation to proliferation. Various types of growth factors [13], cytokines, and chemokines [14] are preferentially secreted in accordance with the changing circumstances during the progression in the wound healing cascade. Growth factors such as bFGF, epithelial growth factor, and hepatocyte growth factor have been clinically utilized and their effects are well-investigated [15, 16]. Growth factors applied topically on wound surfaces stimulate certain cell types to proliferate. Unlike growth factors, G-CSF is a chemokine that stimulates granulocytes and hematopoietic stem cells in the bone marrow. It also regulates neutrophil proliferation, maturation, and survival, and induces inflammatory reactions in wound tissues [17,18]. G-CSF is also known to initiate the mobilisation of not only hematopoietic stem cells but also circulating fibrocytes and EPC, which are involved in granulation tissue formation. However, no increase in circulating CD34+ fibrocytes and EPC was noted in the patients treated with rhG-CSF.
In animal models, recombinant G-CSF administered to haemorrhagic rats with skin wounds accelerates wound healing. This effect was accompanied by an increase in neovascularisation and a decrease in apoptosis [19]. The positive effects of G-CSF on wound healing have been also addressed in clinical studies [8-10, 20]. However, the mechanisms of action through which G-CSF accelerates refractory wound healing are still unclear. In the investigator’s clinical trial, rhG-CSF was topically applied to decubitus skin ulcers of six patients with refractory wounds. Despite the small number of patients, study authors found a statistically significant improvement in wound healing. G-CSF may act by shifting chronic wounds to acute ones and this might involve the activation of indolent neutrophils [21], and neutrophils may play an initial role in the progression of wound healing. However, the involvement of G-CSF in the stimulation of stem cells or progenitor cells to allow regenerative healing could not be verified in this study.
In the present clinical trial, study authors used a unique regimen of drug administration – the direct administration of rhG-CSF on the wound surface. They expected the induction of cell mobilisation from the bone marrow and the stimulation of tissue-resident granulocytes in wound tissue. The former was achieved as evidenced by the increased plasma level of G-CSF and WBC count. On the other hand, the later as a major source of neutrophils could not be determined. However, indolent neutrophils may also pre-exist in chronic wounds and can allow the activation of wound healing reactions. In the investigator’s previous study [22], G-CSF is produced at the site of injury, and G-CSF supplementation to the indolent wounds in diabetic mice accelerated wound healing. The local effects of G-CSF in peripheral tissues should be investigated in a future study.
Previous studies show that systemic G-CSF administration is effective for wounded patients with neutropenia [8-10, 20] and burn wound sepsis in rats [23]. These suggest that its efficacies are due to enhancing neutrophil numbers and/or functions. On the other hand, Iwamoto et al. showed that the systemic administration of G-CSF in mice is effective in the mobilisation of bone marrow stem cells into the circulation, and the cells home to the wound site [24]. In the case of a local administration, the excised wound is more significantly healed than with a systemic injection [25]. These pieces of evidence provide an idea of the dual functions of G-CSF in the body. The first is the classical mobilisation of bone marrow cells such as neutrophils. The second is the local action of G-CSF in wound tissues. Both are highly possible because G-CSF functions as a signal molecule at the site of wounds and acts both locally and systemically. The present results thus suggest the complex mechanisms through which G-CSF can aid in wound healing.
References
- Liarte S, Bernabe-Garcia A, Nicolas FJ (2020) Role of TGF- in Skin Chronic Wounds: A Keratinocyte Perspective. Cells. 9:
- McCarty SM, Cochrane CA, Clegg PD, Percival SL (2012) The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen 20: 125-136.
- Jung K, Covington S, Sen CK, Januszyk M, Kirsner RS, et al (2016) Rapid identification of slow healing wounds. Wound Repair Regen 24: 181-188.
- Tecilazich F, Dinh T, Pradhan-Nabzdyk L, Leal E, Tellechea A , et al (2013) Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One 8: e83314.
- Grieb G, Steffens G, Pallua N, Bernhagen J, Bucala R, et al (2011) Circulating fibrocytes--biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol 291: 1-19.
- Eyles JL, Roberts AW, Metcalf D, Wicks IP (2006) Granulocyte colony-stimulating factor and neutrophils--forgotten mediators of inflammatory disease. Nat Clin Pract Rheumatol 2: 500-510.
- Fuchs A, Monlish DA, Ghosh S, Chang SW, Bochicchio GV, et al (2019) Trauma Induces Emergency Hematopoiesis through IL-1/MyD88-Dependent Production of G-CSF. J Immunol 202: 3020-3032.
- Besner GE, Glick PL, Karp MP, Wang WC, Lobe TE, et al (1992) Recombinant human granulocyte colony-stimulating factor promotes wound healing in a patient with congenital neutropenia. J Pediatr Surg 27: 288-291.
- Cody DT, Funk GF, Wagner D, Gidley PW, Graham SM, et al (1999) The use of granulocyte colony stimulating factor to promote wound healing in a neutropenic patient after head and neck surgery. Head Neck 21: 172-175.
- Fine JD, Manes B, Frangoul H (2015) Systemic granulocyte colony-stimulating factor (G-CSF) enhances wound healing in dystrophic epidermolysis bullosa (DEB): Results of a pilot trial. J Am Acad Dermatol 73: 56-61.
- Bjarnsholt T, Ciofu O, Molin S, Givskov M, Hoiby N , et al (2013) Applying insights from biofilm biology to drug development - can a new approach be developed? Nat Rev Drug Discov 12: 791-808.
- Sanada H, Iizaka S, Matsui Y, Furue M, Tachibana T, et al (2011) Clinical wound assessment using DESIGN-R total score can predict pressure ulcer healing: pooled analysis from two multicenter cohort studies. Wound Repair Regen 19: 559-567.
- Marti-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, et al (2015) Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev 10: CD008548.
- Ridiandries A, Tan JTM, Bursill CA (2018) The Role of Chemokines in Wound Healing. Int J Mol Sci 19: E3217.
- Gospodarowicz D (1990) Fibroblast growth factor. Chemical structure and biologic function. Clin Orthop Relat Res 257: 231-248.
- Akita S, Akino K, Imaizumi T, Hirano A (2005) A basic fibroblast growth factor improved the quality of skin grafting in burn patients. Burns 31: 855-858.
- Panopoulos AD, Watowich SS (2008) Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and 'emergency' hematopoiesis. Cytokine 42: 277-288.
- Roberts AW (2005) G-CSF: a key regulator of neutrophil production, but that's not all! Growth Factors 23: 33-41.
- Huang H, Zhang Q, Liu J, Hao H, Jiang C, et al (2017) Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Wound Healing in Hemorrhagic Shock Rats by Enhancing Angiogenesis and Attenuating Apoptosis. Med Sci Monit 23: 2644-2653.
- Baldelli CM, Ruella M, Scuderi S, Monni M, Passera R, et al (2012) A short course of granulocyte-colony-stimulating factor to accelerate wound repair in patients undergoing surgery for sacrococcygeal pilonidal cyst: proof of concept. Cytotherapy 14: 1101-1109.
- Itoh Y, Kuratsuji T, Tsunawaki S, Aizawa S, Toyama K, et al (1991) In vivo effects of recombinant human granulocyte colony-stimulating factor on normal neutrophil function and membrane effector molecule expression. Int J Hematol 54: 463-469.
- Jimi S, Miyazaki M, Takata T, Ohjimi H, Akita S, et al (2017) Increased drug resistance of meticillin-resistant Staphylococcus aureus biofilms formed on a mouse dermal chip model. J Med Microbiol 66: 542-550.
- Yalcin O, Soybir G, Koksoy F, Kose H, Ozturk R, et al (1997) Effects of granulocyte colony-stimulating factor on bacterial translocation due to burn wound sepsis. Surg Today 27: 154-158.
- Iwamoto S, Lin X, Ramirez R, Carson P, Fiore D, et al. Bone marrow cell mobilization by the systemic use of granulocyte colony-stimulating factor (GCSF) improves wound bed preparation. Int J Low Extrem Wounds 12: 256-264.
- Shen GY, Park IH, Song YS, Joo HW, Lee Y, et al (2016) Local injection of granulocyte-colony stimulating factor accelerates wound healing in a rat excisional wound model. Tissue Eng Regen Med 13: 297-303
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences